COLLAGEN
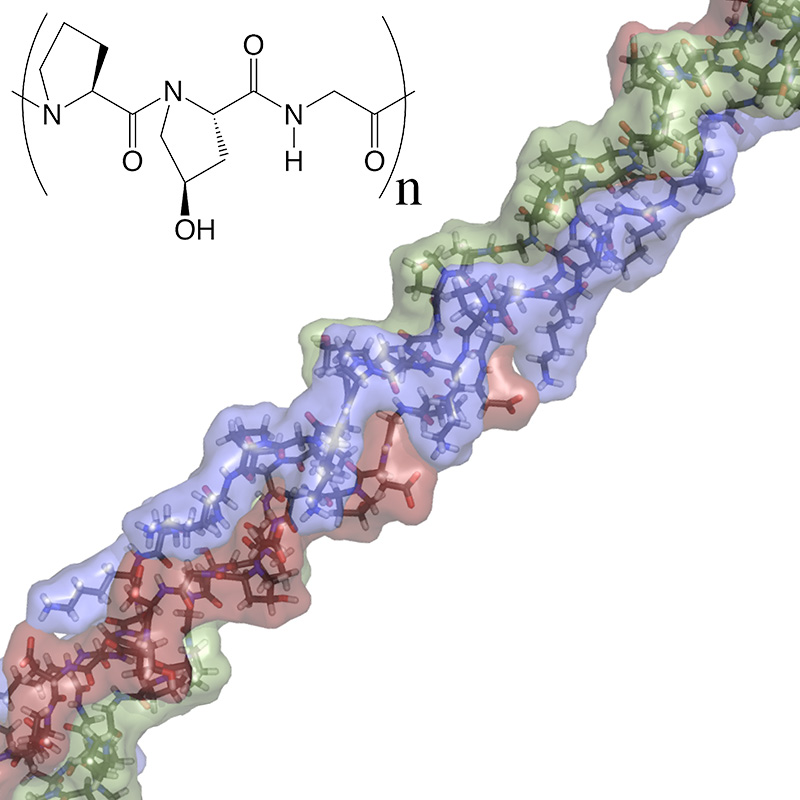
The collagen family of proteins have a fascinating, multi-hierarchical structure and a folding mechanism considerably different from typical globular proteins. This can immediately be seen by the unusual, repeating three amino acid sequence which frequently contains (hydroxyproline-proline-glycine)n. Whereas proline and glycine are typically thought of as structure-breaking amino acids, in collagen they are the keys to a well folded triple helix. Further, hydroxyproline, a post-translationally modified form of proline, is found in great abundance in collagen. These sequences self-assemble to form the most characteristic structure of collagen - the triple helix. Then depending on the type of collagen, these triple helices may continue to assemble to form larger and larger structures. Collagen plays a critical role in tissue organization, mechanical properties and regeneration. Furthermore, the characteristic triple helix of collagen can be found in viruses, bacteria, fungi and eukaryotes. In humans, collagen is by far the most abundant protein by mass. Because of their fascinating and unusual structure, their many important biological properties, their ubiquity across species and abundance in humans, our group has dedicated considerable effort to understand the structure, folding and self-assembly of collagen and collagen-like proteins. Active projects in this area include structure-stability relationships within the triple helix, covalent capture and other cross linking methods, the ability of a synthetic collagen to direct biological function, and the control of synthetic collagen self-assembly into higher order structures and functional biomaterials.
MULTIDOMAIN PEPTIDES
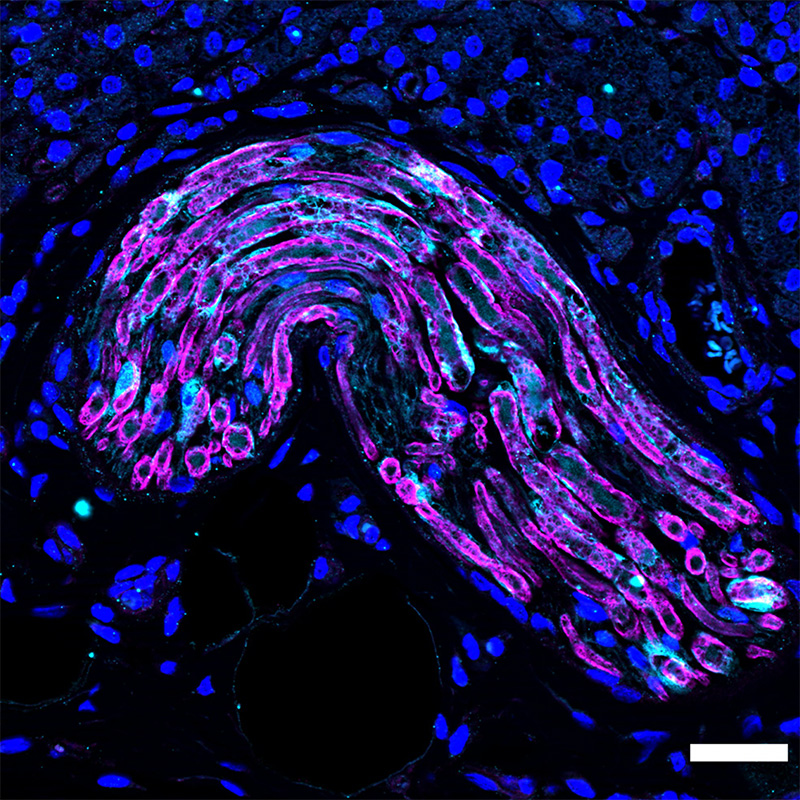
In the protein folding energy landscape, beta-sheet structure is one of the most stable. Consequently it is also one of the most reliable to design and control. Our lab takes advantage of this in our “MultiDomain Peptides”, or MDPs, which are relatively short (~16 amino acid) peptides designed to fold into a beta-sheet conformation that allows for the self-assemble of nanofibers hundreds of nanometers in length while only about 8nm in width. These nano fibers form through the cooperative hydrophobic packing between two adjacent peptide lamellae and amide backbone hydrogen bonding running parallel to the fiber axis. Based on the design of the MDP, the nanofiber assembly is controlled by pH or ionic strength of the aqueous media. At appropriate peptide concentration (usually around 1% by weight) these peptides will self-assemble into nanofibers which subsequently entangle to form hydrogels. We have found that MDPs have remarkable biological and materials properties. For example in vivo these hydrogels are rapidly infiltrated with inflammatory cells which start a cascade of responses that quickly leads to dramatic angiogenesis (blood vessel formation) within the hydrogel. The formation of a suitable blood vessel network is one of the most important hallmarks of a successful tissue regeneration strategy. We are actively pursuing the use of these materials for peripheral nerve regeneration and volumetric muscle loss, diabetic wound healing, control of stem cell behavior, cancer immunotherapy and vaccine development.
Many thanks to the NSF & NIH that make our work possible: NSF-CHE 1709631, NIH-NIDCR R01 DE021798, NIH-NIDCR R01 DE030140.


